Development of Synthetic Platforms for the In Vitro Glycosylation of Proteins
Motivation
N-linked glycans play a role in protein stability, solubility and cell trafficking as well as cell signaling. Therefore, the glycosylation of proteins is one important parameter in the optimization of animal cell culture-derived drugs including monoclonal antibodies, growth factors, and hormones. Over the past years efforts have been made to modify and implement the N-glycosylation machinery in yeast and E. coli, respectively, for the production of biologics with human-type glycoforms. An alternative approach is the in vitro glycoengineering of proteins by synthesizing and modifying the glycostructure via cell-free biocatalytic processes using purified enzymes and nucleotide sugars. Case studies have shown very promising results in terms of increasing the level of galactosylation and sialylation on IgG. In order to in vitro glycosylate proteins, lipid-linked oligosaccharides (LLO) and nucleotide sugars are needed. Challenges associated with their biocatalytic synthesis are, in particular, the expression and the purification of membrane-associated glycosyltransferases, and the provision of expensive sugar nucleotides as building blocks.
Aim of the project
Aim of the project is to develop a cell-free synthetic platform for the efficient synthesis of lipid-linked oligosaccharides for the in vitro glycosylation of proteins. Genes of enzymes used as biocatalysts are expressed in E. coli, S. cerevisiae and Sf9-cells. To engineer multienzyme networks various tools available through the unique infrastructure of the MPI Magdeburg are utilized. These include mathematical modelling (MOD), downstream processing (DSP) as well as state-of-the-art high-throughput glycoanalytics (BPA).
References
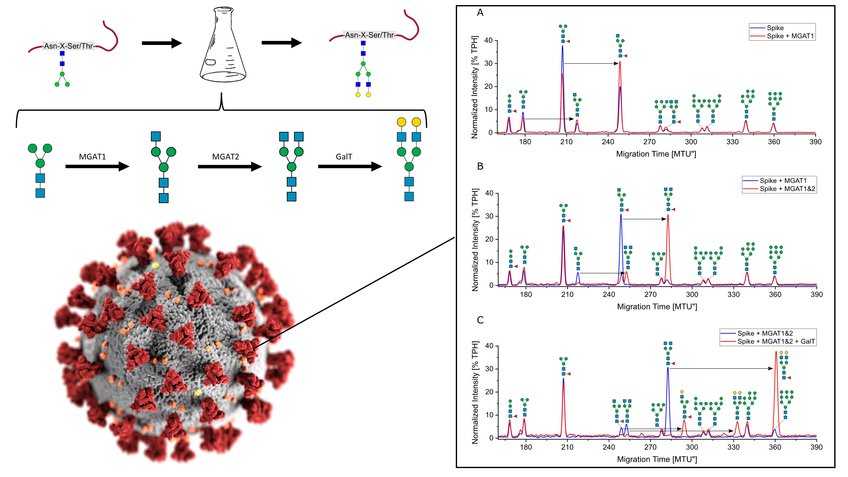
Ruhnau, J.; Grote, V.; Juárez-Osorio, M.; Bruder, D.; Mahour, R.; Rapp, E.; Rexer, T.; Reichl, U.: Cell-Free Glycoengineering of the Recombinant SARS-CoV-2 Spike Glycoprotein. Frontiers in Bioengineering and Biotechnology 9, 699026 (2021)
Rexer, T.; Laaf, D.; Gottschalk, J.; Frohnmeyer, H.; Rapp, E.; Elling, L.: Enzymatic Synthesis of Glycans and Glycoconjugates. In: Advances in Glycobiotechnology, pp. 231 - 280 (Eds. Rapp, E.; Reichl, U.). Springer, Cham, Switzerland (2021)
Rexer, T.; Wenzel, L.; Hoffmann, M.; Tischlik, S.; Bergmann, C.; Grote, V.; Boecker, S.; Bettenbrock, K.; Schildbach, A.; Kottler, R. et al.; Mahour, R.; Rapp, E.; Pietzsch, M.; Reichl, U.: Synthesis of lipid-linked oligosaccharides by a compartmentalized multi-enzyme cascade for the in vitro N-glycosylation of peptides. Journal of Biotechnology
322 (10), pp. 54 - 65 (2020)
Mahour, R.; Klapproth, J.; Rexer, T.; Schildbach, A.; Klamt, S.; Pietzsch, M.; Rapp, E.; Reichl, U.: Establishment of a five-enzyme cell-free cascade for the synthesis of uridine diphosphate N-acetylglucosamine. Journal of Biotechnology 283, pp. 120 - 129 (2018)
Rexer, T.; Schildbach, A.; Klapproth, J.; Schierhorn, A.; Mahour, R.; Pietzsch, M.; Rapp, E.; Reichl, U.: One pot synthesis of GDP-mannose by a multi-enzyme cascade for enzymatic assembly of lipid-linked oligosaccharides. Biotechnology and Bioengineering 115 (1), pp. 192 - 205 (2018)