Downstream Processing
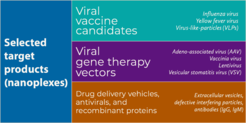
Viral vaccines are one of the most successful achievements of modern science thanks to the hundreds of millions of serious infectious diseases they help prevent. In addition, the use of viral vectors for gene therapy has shown promise to become the next medical revolution for fighting a wide variety of currently untreatable diseases such as hereditary disorders and cancer.
Safe and potent vaccines, and increasingly gene therapies as well, need to be available to a large part of the world population at an affordable price, which represents a huge manufacturing challenge. The wide range of viruses and the huge variety of virus production methods make it extremely difficult to standardize virus purification for vaccine and gene therapy formulations. Moreover, in order to ensure their safety and potency, virus products have to be purified to extremely high standards. All these are considerable challenges for process engineering.
Downstream processing (DSP) refers to the recovery and the purification of biological products. DSP requires several steps and is typically tailored to each virus and how it is produced, making process development and optimization very time-consuming. Furthermore, purification processes that work in the laboratory may not be easily scalable to commercial manufacturing.
Due to process yield, robustness, capacity, scalability, and cost constraints, chromatography is one of the few purification methods than can be used at an industrial scale. However, traditional bead-based chromatography was originally developed for protein purification and often not efficient for DSP of virus particles.
Often there are severe shortages in the supply of vaccines and viral vectors for human use (e.g., clinical trials, commercial supply, pandemic emergencies). In order to meet current and future demands, new and more efficient purification technologies are urgently needed.
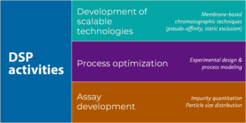
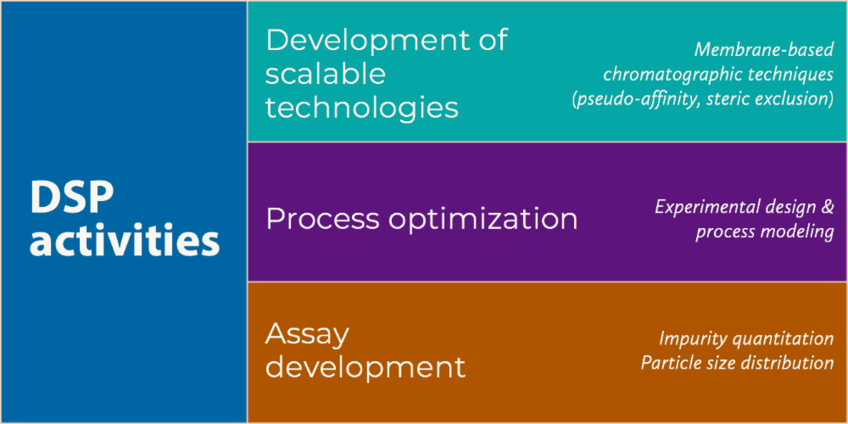
We work on the optimization and the establishment of new DSP methods for the purification of virus particles, focusing strongly on chromatography techniques specifically optimized for large scale manufacturing of biopharmaceutical products. Therefore, we develop efficient purification methods that are fully scalable to industrial production to make life-saving viral treatments affordable and available to the general population.