
A purification platform for viral vaccines & gene therapy vectors
Motivation
Vaccines are considered among the most successful achievements of modern science. Historically made of mostly crude preparations by today’s standards, these products have become more complex as their manufacturing processes have evolved.
The wide range of existing viruses, their production methods, and the emergence of new public health threats make it extremely difficult to homogenize viral vaccine manufacturing. Viruses need to be isolated from complex mixtures and purified (downstream processing) to extremely high standards.
Current methods used in virus downstream processing, however, have to be tailored to each particular virus species and are commonly extremely expensive, time consuming, and result in high product losses.
These challenges are also true for gene therapy, in which huge amounts of viruses are needed to administer a treatment. The high cost of gene therapies, on the range of several hundred thousand dollars, is partially due to the highly ineffective production & purification methods used nowadays. It is estimated that demand for gene therapy vectors outstrips its supply by a factor of 5.
There is a clear need for platform approaches for virus downstream processing. These urgently needed methods should be inexpensive, robust, give high product recoveries, be fully scalable for commercial production, and at best, be tweaked as little as possible for different viruses and processes in order to homogenize virus purification & accelerate process development.
Aim of the project
Our main goal is to develop a one-for-all solution for purifying viruses produced in cell culture.
Using a low-cost stationary phase, e.g., unmodified cellulose, we capture & purify virus particles by steric exclusion chromatography (SXC). In SXC, the crude sample containing the target species is mixed with polyethylene glycol (PEG) and the product is captured without a direct chemical interaction on the non-reactive hydrophilic surface. The purified viruses are then recovered by reducing the PEG concentration in the mobile phase. Selectivity in SXC is strongly influenced by the target species’ size.
This single-use platform chromatography method can be used to purify a wide range of virus’ size and kinds using a very narrow range of operational parameters with extremely high recoveries (>95%). The same conditions are used to purify different strains of a single virus class, making it extremely powerful compared to other techniques.
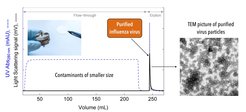
Fig. 1: Membrane-based steric exclusion chromatography (SXC) of influenza virus.
