
Continuous Production of Cell Culture-derived Vaccines in Cascades of Stirred Tank Bioreactors
General motivation
Moving from batch manufacturing to fully continuous virus production can significantly reduce plant downtime, increase process efficiency, reduce plant footprint, and help to reduce the manufacturing costs of viral vaccines and viral vectors [1, 2]. Starting from conventional upstream processing (USP) in batch mode, the establishment of continuous bioreactors for production of cell culture-derived viruses could bring process productivities to a higher level and allow the design of more economical processes. In this mode of operation, fresh medium is added continuously to the bioreactor and a fraction is harvested continuously, while the working volume of the bioreactor is kept constant. Currently, we are exploring two approaches to enable a highly efficient production of cell culture-based viruses in continuous bioreactors, namely cascades of stirred tank bioreactors and a plug-flow tubular bioreactor system.
Motivation for continuous production in cascades of stirred tank bioreactors
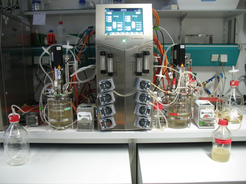
Cascades of two interconnected stirred tank bioreactors (CSTRs, Fig. 1) are an interesting option for continuous production of viral vaccines [3, 4]. In these systems, cell growth and virus propagation are performed in separated stirred tank bioreactors for the production of lytic viruses with a low mutation rate, i.e. modified vaccinia Ankara (MVA) virus [5]. In addition, CSTRs are an option for long term virus passaging allowing detailed studies regarding virus evolution and generation of defective interfering particles (DIPs).
Aim of the project
- Characterization and modeling of a CSTRs for cell culture-based vaccine manufacturing
- Studies regarding the accumulation of defective interfering particles (DIPs), and the evolution of viruses using virus titer measurements, qualitative and quantitative PCR, and flow cytometry
![Fig. 2 Experimental set-up of a continuous process for virus production using two interconnected stirred tank bioreactors [3,4,5].](/3381132/original-1518440975.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMzODExMzJ9--c3112704effdff0aca0e487a436b49d61c1c8afd)
Fig. 2 Experimental set-up of a continuous process for virus production using two interconnected stirred tank bioreactors [3,4,5].

![Fig. 2 Experimental set-up of a continuous process for virus production using two interconnected stirred tank bioreactors [3,4,5]. Fig. 2 Experimental set-up of a continuous process for virus production using two interconnected stirred tank bioreactors [3,4,5].](/3381132/original-1518440975.jpg?t=eyJ3aWR0aCI6MzQxLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzM4MTEzMn0%3D--53d0014731a5dc373241b22b2a6e90d13244c0cd)