
Scouting for new virus production options
Motivation
Processes for recombinant protein production (i.e. monoclonal antibodies) are giving the lead regarding cell line optimization, medium design, and process intensification using fed-batch or perfusion cultures. Viral vaccine industry, however, is slow in implementation of similar technologies. Therefore, our group is scouting on new virus production options not only for “established” vaccines but also for new vaccines for emerging diseases or for therapeutic approaches. With the aim to modernize vaccine manufacturing a knowledge base is build-up for processes using suspension and adherent cells. For this, a wide range of cultivation vessels and process strategies, i.e. fed-batch, perfusion and continuous systems, are evaluated for production of a selected set of viruses including influenza A virus, MVA virus, flaviviruses and oncolytic fusogenic viruses.
Aim of the project
For this concept, small scale experiments using cell culture flasks up to 10 L benchtop bioreactors are evaluated for suspension cells or packed bed reactors for adherent cells using either fed-batch, perfusion or continuous operation modes. Process characterization involves the use of conventional and state-of-the art sensors for on- and off-line monitoring and the analysis of cell growth, cell metabolism, and virus replication (virus titration, flow cytometry for infection status, defective particles). Our final aim is to establish a knowledge base for intensified virus and viral vector production.
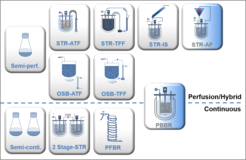
Towards higher cell densities...
To accomplish this strategy, many different parameters have to be analyzed experimentally and integrated into mathematical models (pH, pO2, on-line capacitance, glucose, lactate & ammonium concentration, cell growth rate, virus titer, ...). Furthermore, advanced analytical methods such as flow cytometry to characterize the properties of cell populations, or HPLC methods to determine the concentration of intracellular metabolites and specific enzyme activities during cell growth and virus replication are being used.
Variations of cell culture medium (serum-free, chemically defined), bioreactors (disposable, stirred versus orbital shaken (OSB)), viruses (influenza A and B, flaviviruses, MVA) and host cells (MDCK, HEK293, BHK21, Vero, AGE1.CR, etc.) are evaluated in-depth for the characterization of different process conditions. Cultivation of suspension cells with perfusion results in cell concentrations of more than 8 x 107 cells/mL. We currently focus on disposable hollow fiber technology (HFBR, ATF, TFF, TFDF) as well as inclined settlers or acoustic filter systems for perfusion cultures aiming not only for high cell-specific virus yields but also for smooth integration of subsequent downstream processing steps. If useful, we evaluate first steps of downstream processing optimizing harvest time point or reduction of host cell protein (HCP) and host cell DNA.
Towards higher continuous virus production...
Infecting constantly new cells that are added to the process and constantly removing virus that is produced by the cells could lead to continuous virus manufacturing processes. Two-stage stirred tank bioreactors, both running in continuous mode with a cell reactor feeding the virus vessel is one option. Alternatively, a tubular plug flow reactor (PFBR) with an infection of cells at the beginning of the tube that then flow through the tube with a given residence time could be used. Evaluating and optimizing both systems for various virus-host cell systems will complete our knowledge base and also help to better understand the generation of defective interfering particles in these systems.

Outlook
Fine tuning of these systems can be done with additional knowledge on intracellular metabolites, signalling, optimization of medium composition (serum-free, protein-free, chemically defined, virus booster, viral sensitizers), virus seed generation and virus-host cell interaction (reverse genetics), virus replication dynamics or cellular physiology (flow cytometry). Implementing AI (artificial intelligence) or setting up digital twins will help to further improve these processes.

