Model-based analysis of chemotherapy-induced peripheral neuropathy (CIPN)
CIPN is an unwanted side-effect of a number of chemotherapy drugs for cancer treatment. It leads to pain in the outer extremities and can therefore result in dose limitations or even termination of the chemotherapy in extreme cases.
In this project we study the electrical signaling on the level of a single sensory neuron to gain a deeper understanding of the pathophysiology of pain and how this is affected by chemotherapy [1]. This is a joint research activity with Prof. Ramkrishna, Prof. Yang Yang and Parul Verma from Purdue University. Parul joined the MPI for several longer stays during her PhD to work with us on this project.
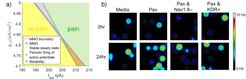
The approach is based on mathematical Hodgkin Huxley-type models accounting for additional relevant ion channels. It uses methods from stability and bifurcation theory, which has been a particular field of expertise in our group over the years with main focus on chemical systems, and which has been further strengthened by former MPI senior researcher Prof. Flockerzi. A characteristic result from [2] is shown in Fig. 1a. The figure nicely summarizes different patterns of behavior depending on a sensitive sodium channel conductivity and some external current. In the ‘no pain’ region the cell potential is settling down to a stable steady state, whereas in the ‘pain’ region periodic firing of action potentials occurs. In the upper part of the diagram there is a graded transient between the ‘no pain’ and the ‘pain’ region via mixed mode oscillations (MMOs) consisting of 1 action potential and N small sub threshold peaks. The number of sub threshold peaks in the MMO region is increasing step-by-step from right to left and the firing rate of action potentials, which is related to the intensity of pain, is decreasing accordingly. In between, fascinating concatenated MMOs can be observed. It was shown that they can be described elegantly using Farey arithmetics from number theory [2]. In contrast to this, in the lower region there is a sharp transient between the ‘pain’ and ‘no pain’ region which is associated with hysteresis and bistability.
The right diagram in Fig. 1b shows a first experimental validation obtained by the Yang Yang group at Purdue [3]. Using multi-electrode array recordings, it has been shown that the firing rate is highest for medium doses of the cancer drug paclitaxel and can be reduced by a Nav 1.8 channel blocker or KDR+ channel enhancer as predicted qualitatively by the mathematical model.
Cooperation partners
- Prof. Ramkrishna, Purdue University
- Prof. Yang Yang, Purdue University
- Dr. Parul Verma, Purdue University
Publications
[1] P. Verma, A. Kienle, D. Flockerzi, and D. Ramkrishna. Using bifurcation theory for exploring pain. Ind. Eng. Chem. Res., 59:2524–2535, 2020.
[2] P. Verma, A. Kienle, D. Flockerzi, and D. Ramkrishna. Computational analysis of a 9-D model for a small DRG neuron. J. Comput. Neurosci., 2020. doi:10.1007/s10827-020-00761-6.
[3] P. Verma, M. Eaton, A. Kienle, D. Flockerzi, Y. Yang, and D. Ramkrishna. A mathematical investigation of chemotherapy-induced peripheral neuropathy. Front. Comput. Neurosci., 2020. doi: 10.3389/fncom.2020.564980.
