Control of Methanol Synthesis
Introduction

Methanol is an important basic chemical in the chemical industry. It is produced in large amounts under steady state conditions from synthesis gas using Cu/ZnO/Al2O3 catalysts. With the forthcoming “energy revolution” it is also becoming an important energy carrier. Excess wind or solar electrical energy can be converted to hydrogen and react with CO and CO2 to produce methanol for chemical energy storage. Typical sources of CO and CO2 are biomass and waste streams with variable compositions. Methanol can be used as a fuel, fuel additive or starting material for dimethyl ether synthesis as a promising substitute for diesel fuels. Alternatively, it can be converted back to electricity using fuel cell technology. Due to unavoidable fluctuations in the available resources, dynamic operation of methanol synthesis becomes a major issue.
Approach and Results
In this project, we develop model based control strategies for dynamic operation of methanol synthesis. This implies process control and process management under unavoidable external fluctuations, but also novel forced periodic operational strategies to improve methanol yields. The latter is done in close cooperation with the PCF group and Prof. Menka Petkovska from Belgrade University, who is a leading specialist in nonlinear frequency response analysis of chemical processes.
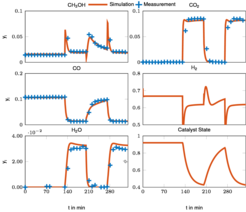
As a starting point, new kinetic models for methanol synthesis under strongly varying feed conditions were developed. They are based on a comprehensive set of steady state and dynamic data obtained previously by the PCF group [1] and account for dynamic changes in the catalyst morphology, which play an important role under transient conditions. It turned out that a detailed model based on 22 elementary reaction steps which was already proposed in similar form in [1] is not identifiable with the available measurement information. Therefore, a lumping of reaction steps was introduced. Global deterministic optimization was applied to determine the parameters and to avoid suboptimal local solutions.
The lumped model is able to describe the transient behavior over a wide range of operating conditions as illustrated in Fig. 1 [2]. In a subsequent work the impact of catalyst deactivation over time, as illustrated in Fig. 2, was investigated, which lead to an extension of the lumped kinetic model with a deactivation model [3].
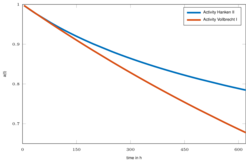
Figure 2: Catalyst deactivation over experiment duration
Future Work
The developed model will be further refined and validated in close cooperation with the PCF group. Optimal experimental design will be applied to calculate optimal dynamic stimuli for rigorous parameter identification. The model will then serve for rigorous optimization of dynamic reactor operation under random fluctuations arising in chemical energy storage. Embedded in the new DFG priority program SPP 2080 “Catalysts and reactors under dynamic conditions for energy storage and conversion" options for methanol synthesis under forced periodic operation conditions will be evaluated in cooperation with the PCF group and Prof. Menka Petkovska from university Belgrade.
Funding
This project is embedded in the national priority program SPP 2080 with funding from DFG.
Cooperation partners
- Prof. Seidel-Morgenstern PCF group
- Prof. Menka Petkovska University Belgrade
Publications
[1] B. Vollbrecht, Zur Kinetik der Methanolsynthese an einem technischen Cu/ZnO/Al2O3-Katalysator, PhD thesis, Otto-von-Guericke-Universität Magdeburg, 2007.
[2] C. Seidel, A. Jörke, B. Vollbrecht , A. Seidel-Morgenstern, A. Kienle , Kinetic modeling of methanol synthesis from renewable resources, Chemical Engineering Science, 175 (2018), pp. 130–138.
[3] C. Seidel, A. Jörke, B. Vollbrecht, A. Seidel-Morgenstern, A. Kienle, Kinetic Modeling of Methanol Synthesis - Impact of Catalyst Deactivation, in Computer Aided Chemical Engineering, vol. 43, 2018, pp. 85–90.


