Affinity Chromatography of Influenza Virus
Affinity chromatography is a method of selectively and reversibly binding target compounds e.g. proteins to immobilized molecules on solid support matrices based on the exploitation of known physico-chemical and biological affinities between the interacting molecules. The interaction is based on structural properties, such as the interaction of antibodies with antigens, lectins with carbohydrates, enzymes with substrate analoges, hormones with receptors, and nucleic acids with binding proteins.
In our laboratory we are designing and optimizing different affinity matrices for the downstream processing of cell culture based (Madin Darby canine kidney cells) influenza vaccines. Currently studied affinity interactions are centered on lectins, antibodies and chemical analoges of enzymatic substrates.
Lectin-affinity chromatography (LAC) is based on a selective affinity of lectins, which are carbohydrate specific proteins of non-immune origin, for a carbohydrate or a group of carbohydrates. The influenza A virus contains two spike glycoproteins on its surface: hemagglutinin (HA) and neuraminidase (NA). HA is the most abundant surface protein and also the main antigenic protein of the influenza A virus. It is a trimeric glycoprotein containing per subunit 3 to 9 N-linked glycosylation sites depending on the viral strain. Detailed analysis of these sites and their individual glycan structures are presently performed in house.
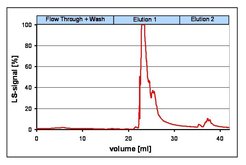
Lectins immobilized on different column and membrane based supports have been successfully applied for the capturing of various influenza virus strains from MDCK cell culture broths. Thereby contaminating host cell nucleic acid as well as proteins could be highly reduced while virions were concentrated.
Literature:
Opitz, L., Salaklang, J., Büttner, H., Reichl, U., Wolff, M.W. 2007. Lectin-affinity chromatography for downstream processing of MDCK cell culture derived human influenza A viruses. Vaccine 25: 939-947.
Opitz, L., Lehmann, S., Zimmermann, A., Reichl, U., Wolff, M.W. 2007. Impact of adsorbents selection on capture efficiency of cell culture derived human influenza viruses. Journal of Biotechnology 131: 309-317.
Wolff, M.W., Opitz, L., Reichl, U. Method for purification of viral proteins. European Patent: Application number: EP07007759, Application date: 17.04.2007 USPTO: Application number: US 60/912,305; Application date: 17.04.2007
Interconnections with other projects of Bioprocess Engineering
Coupled Processes:
Influenza Vaccine Production in Microcarrier Systems
Downstream Processing of Influenza Virus
Particulate formulation of influenza virus antigen

