
ContiVir Spinoff – Enabling bedside manufacturing of viral vaccines and cell & gene therapies
Motivation
Viruses are the basis for the production of many vaccines and cell & gene therapies (CGT) and their efficient manufacture is key for the successful commercialization and adoption of these treatments.
There is currently a serious lack of supply of virus particles partly due to limited manufacturing capacity by a handful of facilities worldwide that mainly rely on complicated, slow, and inefficient batch methods. This known issue has been publicly expressed by several industry experts and biopharmaceutical companies.
With a growing number of vaccines and CGTs approved in the next years, a still increasing world population, and the emergence of serious public health threats such as COVID-19 that highly disrupt the biopharmaceutical supply chain, experts agree that incremental changes in production capacity with current technologies will not be enough to cope with the huge demand for virus manufacturing.
It is estimated that a tenfold improvement in virus manufacturing is needed to enable the growth of the gene therapy market ($11 bn by 2025). New, highly efficient technologies that address these issues are urgently needed.
ContiVir is developing highly efficient virus production technologies than can be used for a wide range of virus-based products (around half of the current coronavirus vaccine candidates) and that have the potential to resolve the issues above by simplifying, miniaturizing, and de-centralizing manufacturing.
Aim of the Spinoff
The democratization of virus manufacturing technologies is ContiVir's main motivation.
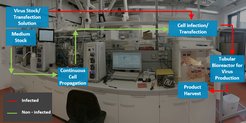
Hence, ContiVir's long-term goal is to develop a more flexible, cost-efficient, and miniaturized factory (microfactory). As a first step toward this goal, ContiVir’s current work focuses on the development and construction of a continuous tubular bioreactor (CTB, Figure 1) and a steric exclusion purification column (SXC, Figure 2) to GMP standards production of clinical material for humans.
Both technologies were developed within the Bioprocess Engineering Group headed Prof. Udo Reichl at the Max Planck Institute in Magdeburg.
ContiVir’s research is funded by the European Union and the German federal government with a 1.6m€ EXIST Forschungstransfer grant. ContiVir’s laboratory facilities are located at the Chair for Bioprocess Engineering at the Otto von Guericke University Magdeburg.
More information at www.contivir.com

Selected References
All publications can be found in the ContiVir’s project website: www.contivir.com/publications

