Bottom-up assembly of a light-driven ATP regeneration module
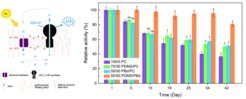
In this project, principles of photophosphorylation are mimicked in order to bottom up assemble light-to ATP artificial organelles (Fig. 1 left). They employ a complex transmembrane enzyme ATP synthase inserted in membranes of nano-sized compartments in the form of vesicles for ATP synthesis. The latter is driven by the proton gradient, which is established and maintained via the proton translocation of light-driven proton pump (bacteriorhodopsin). Our recent focus was on long term stability [1] (Fig. 1 right) of light-to-ATP artificial organelles as well as on integration of these organelles with other life like functions (multistimuli adhesion responsive module [2] and motility [3]). In cooperation with Professor Wagner’s group (University of Münster) we combined a multi-stimuli sensitive adhesion unit with a light-driven energy regeneration module [2]. Integration of the adhesion unit with the ATP conversion module into a single synthetic cell allowed it to adhere to surfaces under blue light illumination, non-oxidative conditions, at neutral pH, and in the presence of metal ions, which were the right conditions for ATP synthesis. Thus, the multi-stimuli responsive adhesion unit allowed self-positioning of the synthetic cell and execution of its functions. Furthermore, in cooperation with MPI Göttingen, we constructed a prototype of synthetic swimmers [3] by combining our light-switchable energy module with demembranated flagella – minimal motility machinery. The ATP production by the energy module facilitated efficient propulsion of the cargo attached to flagella, paving the way for future development of advanced targeted drug delivery carriers. We expect that our developed scheme of circulating energy consumption and production could serve as a potential platform for driving the restructuring of other filamentous biopolymer networks (actin,microtubules, and various regulatory components), thus facilitating morphological deformations of an artificial cell.
Funding: BMBF / MaxSynBio
Collaborations:
Prof. Sundmacher & Dr. Ivanov, MPI Magdeburg
Prof. Börsch, U. Jena
Prof. Wegner, University of Münster
Prof. Bodenschatz & Dr. Gholami, MPI for Dynamics and Self-Organization (MPIDS), Göttingen
Selected recent publications
[1] Kleineberg, C., Wölfer, C., Abbasnia, A., Pischel, D., Bednarz, C., Ivanov, I., Heitkamp, T., Börsch, M., Sundmacher, K., and Vidaković-Koch, T., Light-Driven ATP Regeneration in Diblock/Grafted Hybrid Vesicles. ChemBioChem, 2020. 21(15): p.2149-2160.
[2] Xu, D., Kleineberg, C., Vidaković-Koch, T., and Wegner, S.V., Multistimuli Sensing Adhesion Unit for the Self-Positioning of Minimal Synthetic Cells. Small, 2020. 16(35): p. 2002440.
[3] Ahmad, R., Kleineberg, C., Nasirimarekani, V., Su, Y.-J., Goli Pozveh, S., Bae, A., Sundmacher, K., Bodenschatz, E., Guido, I., Vidaković-koch, T., and Gholami, A., Light-Powered Reactivation of Flagella and Contraction of Microtubule Networks:Toward Building an Artificial Cell. ACS Synthetic Biology, 2021. 10(6): p. 1490-1504.
