Max-DePoly: Energy-efficient recycling of polyamides
The use of industrial plastic products is extensive because of their low production cost, valuable chemical and physical properties, and their durability in use. After the use phase, however, the majority of the collected plastic residues are typically burned for energy supply or disposed in a landfill. The Max-DePoly project is based on the emerging idea of the closed circular economy: plastics are manufactured, used and subsequently chemically depolymerized to monomers. The chemical recycling to monomers (CRM) enables a complete reuse of polymeric materials to produce again high quality polymers without loss of quality in their properties.
The Max-DePoly research project focuses on the depolymerization of polyamides (PA). Due to their extreme high durability and strength, various polyamide categories are used in sportswear, textiles, carpets, lightweight vehicle construction and in 3D printing. The PA polymers are important thermoplastics that are produced by condensation reactions in which the amide bonds (-NH-C=O-) connect the repeating units in the polymer backbone with one another (see Figure 1).

Due to the internal hydrogen bonding in the polymer crystal structure, the amide bonds result in exceptional stability for the structure. The Max-DePoly project considers ionic liquids (IL) as unconventional solvents for the depolymerization of the PA fibers.
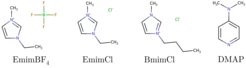
Ionic liquids are low-melting liquid salts with organic cations and suitable anions with exceptional solvation capabilities. Suitable ionic liquids with specific solvation properties are to be identified in order to break the hydrogen bond between the polymer chains and to decompose the crystalline PA structure. The molecular interaction of asymmetric and flexible ions of ionic liquids with very different size and shape (see Fig. 2) facilitates polymer decomposition. Since ionic liquids have a very low vapor pressure, liquid-liquid extraction is the most suitable technique for separating the obtained monomers from an ionic liquid after the reaction.
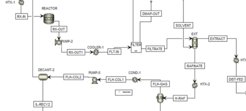
The model-based design and optimization of an energy-optimized and sustainable depolymerization process is another important goal of the project (see Fig. 3). The overall process optimization includes a technically feasible recovery strategy of the purified monomers as well as a smart recycling of the used ionic liquids back to the reaction step.


