
ProPhoS: Biochemical Production Systems using Photosynthetic Organisms
Photosynthetic microorganisms such as microalgae are innovative cell factories for the environmentally benign production of many bio-based chemicals. Such microorganisms are capable to synthesize a wide range of macromolecules, e.g. lipids, carbohydrates, proteins and valuable secondary metabolites from sunlight, carbon dioxide and inorganic nutrients at higher volumetric and areal productivities compared to terrestrial crops. As examples for photosynthetic systems, we have focused our research so far on the β-carotene producing green microalga Dunaliella salina and the siliceous diatom Phaeodactylum tricornutum.
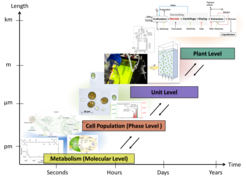
Modelling microalgae processing is a challenging task: biological cell populations represent a complex dynamic system hierarchically organized on multiple temporal and spatial scales of which we have only limited mechanistic knowledge (Fig. 1).
A typical microalgal process (Fig. 2) starts with the cultivation of the microorganisms. Given the hierarchical multi-scale structure of a microalgae cultivation, the Elementary Process Functions (EPF) Methodology is ideally suited for the optimal design of bioreactors. Therefore, we have developed computational models to describe both cellular metabolism and the bioreactor dynamics, and advanced experimental methods to validate our findings [1, 2, 3, 4]. Using the computational models, we were able to identify optimal operating conditions for the cultivation of D. salina with respect to various objectives.
After cultivation, harvest of the microalgae follows. We investigated chemical and physical flocculation as an innovative β-carotene harvesting approach for D. salina [5]. On the plant level, an overall process model based on mass and energy balances was used to compare alternative optimal routes from the individual cell and cell culture down to the final product [6]. Different harvesting methods were compared by integrating all experimental results into the process model [5].
In industrial processes the harvested biomass is dried before the target molecules are extracted. To recover β-carotene from dried D. salina, we found out that supercritical CO2 extraction is a promising sustainable method [7]. However, the biomass drying remained as energy intensive bottleneck when we consider the overall microalgal process.
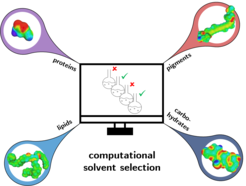
Currently, we are focusing our investigations towards innovative extraction strategies coupled with rational solvent selection for wet algal paste in order to omit the energy-intense drying step (Fig. 3). This promising solvent strategy aims to extract and separate all biomass fractions, as we are fully convinced of the importance to valorize all macromolecular fractions in algal biomass in line with the idea of a zero-waste biorefinery [8, 9, 10].
The algal carbohydrates are one of the main cell constituents in the remnant. Therefore, we also developed new types of heterogeneously catalyzed conversion steps for the exhaustive valorization of the remnant fractions. Here, we successfully converted the carbohydrates to key renewable molecules, e.g. 5-hydroxy methyl furfural and levulinic acid [11]. As known from the sugar chemistry, the yield of 5-HMF could be significantly increased by use of optimized biphasic solvent systems [12].
![Fig 2: Flow sheet of a microalgal process. The PSE group has several contributions for microalgal cell level biochemistry [4] and cultivation [1, 2, 3], harvest [5, 6] and extraction unit [7]. Currently, we focus on developing innovative downstream routes to eliminate the energy-intense drying step [8, 9]. We aim to valorize all macromolecular biomass fractions, e.g. we successfully converted oil-free remnant biomass to the platform molecules 5-HMF and levulinic acid [10, 11].](/4238213/original-1637249344.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjQyMzgyMTN9--caf890158e3cdf7ce797ab6254e4397917559cd7)
Public Outreach activity: https://youtu.be/Ax2s-U_QzuY
Publications
[1] Fachet, M.; Flassig, R.; Rihko-Struckmann, L.; Sundmacher, K.: Carotenoid Production Process Using Green Microalgae of the Dunaliella Genus: Model-Based Analysis of Interspecies Variability. Industrial and Engineering Chemistry Research 56 (45), pp. 12888 - 12898 (2017)
[2] Fachet, M.; Hermsdorf, D.; Rihko-Struckmann, L.; Sundmacher, K.: Flow cytometry enables dynamic tracking of algal stress response: A case study using carotenogenesis in Dunaliella salina. Algal Research (13), pp. 227 - 234 (2016)
[3] Flassig, R.; Fachet, M.; Höffner, K.; Barton, P. I.; Sundmacher, K.: Dynamic flux balance modeling to increase the production of high-value compounds in green microalgae. Biotechnology for Biofuels 9 (165), pp. 1 - 12 (2016)
[4] Fachet, M.; Witte, C.; Flassig, R.; Rihko-Struckmann, L.; McKie-Krisberg, Z.; Polle, J.; Sundmacher, K.: Reconstruction and analysis of a carbon-core metabolic network for Dunaliella salina. BMC Bioinformatics, 21: 1 (2020)
[5] Pirwitz, K.; Flassig, R.; Rihko-Struckmann, L.; Sundmacher, K.: Energy and operating cost assessment of competing harvesting methods for D. salina in a β-carotene production process. Algal Research 12, pp. 161 - 169 (2015)
[6] Pirwitz, K.; Rihko-Struckmann, L.; Sundmacher, K.: Comparison of flocculation methods for harvesting Dunaliella. Bioresource Technology 196, pp. 145 - 152 (2015)
[7] Ludwig, K.; Rihko-Struckmann, L.; Brinitzer, G.; Unkelbach, G.; Sundmacher, K.: β-Carotene extraction from Dunaliella salina by supercritical CO2. Journal of Applied Phycology 33, pp. 1435–1445 (2021)
[8] König-Mattern L.; Rihko-Struckmann L.; Linke S.; Sundmacher K.: Computational solvent screening for efficient microalgal-based biorefineries exemplified by Phaeodactylum tricornutum. International Conference on Algal Biomass, Biofuels and Bioproducts (2021), virtual
[9] König-Mattern L.; Rihko-Struckmann L.; Linke S.; Sundmacher K.: Computational Solvent Screening for the Fractionation of Wet Microalgal Biomass Exemplified by Phaeodactylum tricornutum. 13th European Congress of Chemical Engineering and 6th European Congress of Applied Biotechnology, (2021), virtual
[10] König-Mattern, L.; Linke, S.; Rihko-Struckmann, L.; Sundmacher, K. Computer-Aided Solvent Screening for the Fractionation of Wet Microalgae Biomass. Green Chemistry (2021), 10.1039.D1GC03471E.
[11] Rihko-Struckmann, L.; Molnar, M.; Pirwitz, K.; Fachet, M.; McBride, K.; Zinser, A.; Sundmacher, K.: Recovery and Separation of Carbohydrate Derivatives from the Lipid Extracted Alga Dunaliella by Mild Liquefaction. ACS Sustainable Chemistry & Engineering 5 (1), pp. 588 - 595 (2017)
[12] Rihko-Struckmann, L.; Oluyinka, O. B.; Sahni, A.; McBride, K.; Fachet, M.; Ludwig, K; Sundmacher, K.: Transformation of remnant algal biomass to 5-HMF and levulinic acid: influence of a biphasic solvent system. RSC Advances 10 (42), pp. 24753–24763 (2020)

![Fig 2: Flow sheet of a microalgal process. The PSE group has several contributions for microalgal cell level biochemistry [4] and cultivation [1, 2, 3], harvest [5, 6] and extraction unit [7]. Currently, we focus on developing innovative downstream routes to eliminate the energy-intense drying step [8, 9]. We aim to valorize all macromolecular biomass fractions, e.g. we successfully converted oil-free remnant biomass to the platform molecules 5-HMF and levulinic acid [10, 11]. Fig 2: Flow sheet of a microalgal process. The PSE group has several contributions for microalgal cell level biochemistry [4] and cultivation [1, 2, 3], harvest [5, 6] and extraction unit [7]. Currently, we focus on developing innovative downstream routes to eliminate the energy-intense drying step [8, 9]. We aim to valorize all macromolecular biomass fractions, e.g. we successfully converted oil-free remnant biomass to the platform molecules 5-HMF and levulinic acid [10, 11].](/4238213/original-1637249344.jpg?t=eyJ3aWR0aCI6MzQxLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6NDIzODIxM30%3D--213633fc787bc6708a7191c2e2a5df41c374f9e8)
