DIP VAK - Defective interfering particles as antivirals and live vaccines
Motivation
The influenza A virus (IAV) is a major human pathogen that causes regular annual epidemics. When novel strains emerge, IAV infections can also cause a severe pandemic, which is considered as an imminent threat. Annual vaccination is the most important measure to prevent seasonal influenza infection. In addition, to treat acute infections, small molecule drug antivirals have been developed and approved for the treatment of acute infections. However, circulating human IAV strains have acquired resistance to many commonly used antivirals. Therefore, new antiviral treatment options are urgently needed.
In this research and development project, defective interfering particles (DIPs) of IAV are proposed as a promising new class of antiviral agents. We and other research groups have demonstrated a strong antiviral efficacy of DIPs against IAV infection in various animal models including mice and ferrets. DIPs could be administered intranasally as a droplet spray to the initial site of infection (pharynx) as a prophylactic and/or therapeutic antiviral agent for human use (Fig. 1).
DIPs are naturally occurring, replication-deficient viral particles that exert an inhibitory effect on replication and spread of the wild-type, infectious IAV by hijacking viral and cellular resources. IAV DIPs typically suppress a wide range of IAV strains including current human epidemic, pandemic and even highly pathogenic avian IAV. Surprisingly, IAV DIPs can also suppress non-homologous viral replication, including that of several respiratory viruses such as respiratory syncytial virus (RSV) and SARS-CoV-2. This non-specific inhibition is inherent to IAV DIPs due to their ability to stimulate the innate immunity and to establish a so-called antiviral state. Together, this suggests that IAV DIPs could be used as broadly-acting antiviral agents to treat respiratory viral infections as a fast countermeasure to protect people at risk and restrict virus spread, e.g., in the event of a pandemic.
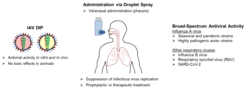
Aim of the project
A new type of IAV DIP discovered in our laboratory, designated “OP7”, will be used as a starting point to generate “improved” DIPs. Specifically, we will use a plasmid-based reverse genetics workflow to genetically engineer OP7 DIPs in order to improve their potency. In addition, IAV DIPs will be engineered for use as a mucosal vaccine, i.e. as a live intranasal influenza vaccine, to address the need for the development of novel mucosal vaccines, as called for by experts. The safety, immunogenicity and antiviral efficacy of the new constructs will be investigated in mice in collaboration with the Bruder lab (Helmholtz Centre for Infection Research, Braunschweig, Germany).
Furthermore, research will be conducted on the mechanism of action of IAV DIPs to establish acceptance of this new class of antivirals/vaccines for medical treatment and to alleviate regulatory matters in the later approval process of this new treatment strategy.
To this end, we have established cell and DIP seed virus banks in according to good manufacturing practice (GMP) guidelines in collaboration with the Fraunhofer Institute for Toxicology and Experimental Medicine (Braunschweig, Germany). This initiative is pivotal in attracting pharmaceutical companies to license our technology and IP with the objective of advancing this novel class of antivirals/live vaccines towards preclinical and phase I clinical studies to improve pandemic preparedness.


