Reaction network analysis and kinetics
Catalyst preparation, kinetic characterization and modulation of deactivation effects
The demand for propene has been growing over the past years as propene is used to produce a variety of chemicals (e.g. polypropylene). Propene is obtained mainly from naphtha steam crackers as a co-product to ethene, and as a co-product from gasoline production from fluid catalytic cracking (FCC) units. One of the potential alternatives to deal with the increasing gap between demand and supply is the direct conversion of ethene to propene (ETP-reaction) by using a catalyst constituted of both MCM-41 and AlMCM-41 doped with nickel. This catalytic system is efficient but faces a severe drawback with fast deactivation with time on stream. Therefore, an alternative is investigated in form of a sequential reactor setup.
Ethene to propene (ETP): Catalyst preparation and process concepts
Propene is a central building block for the chemical industry. One promising on-demand alternative to increase the availability of propene is the direct conversion of ethene.
3 C2H4 --> 2 C3H6 (ETP- reaction)
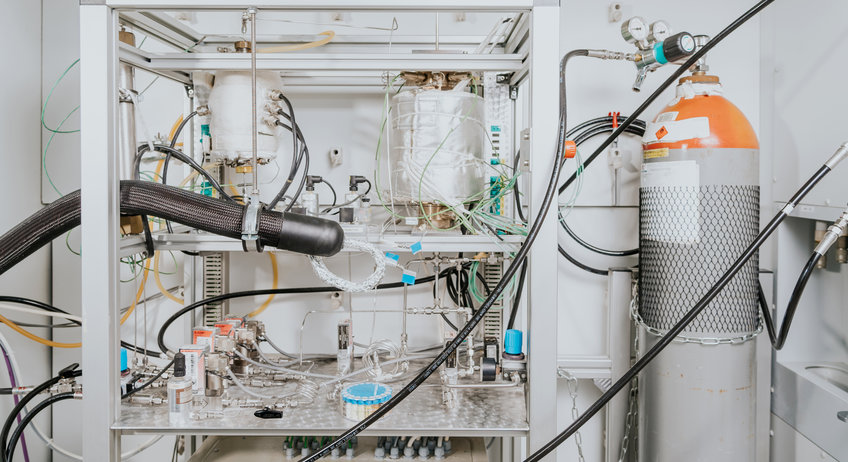
This reaction proceeds via a reaction network, consisting of three consecutive reactions, as show in Figure 1 and given in detail below.
Reaction network
- Dimerization of ethene (Ni/AlMCM-41 and acid sites) (Cat. 1)
- Isomerization of 1-butene (Acid sites) (Cat. 1)
- Metathesis of 2-butene and ethene (NiRe-, Re-, Mo-, W- based catalyst) (Cat. 2)
![Figure 1: ETP reaction mechanism.[Felischak, M., Wolff, T., Alvarado Perea, L., Seidel-Morgenstern, A., & Hamel, C. (2019). Influence of process parameters on single bed Ni/(Al)MCM-41 for the production of propene from ethene feedstock. Chemical Engineering Science, 210: 115246.]](/3553075/original-1636650272.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjM1NTMwNzV9--da39cac390d4c2b1829de18467005d0797d7f171)
[Felischak, M., Wolff, T., Alvarado Perea, L., Seidel-Morgenstern, A., & Hamel, C. (2019). Influence of process parameters on single bed Ni/(Al)MCM-41 for the production of propene from ethene feedstock. Chemical Engineering Science, 210: 115246.]
The Ni/AlMCM-41 material is an interesting catalyst, capable of performing all three reaction steps at once, which has been developed previously. In the process of intensification of the propene production, a reasonable attempt is the decoupling of the ongoing reaction network.
Project goal
Realization of a two-reactor concept (see Figure 2) with the separation of the metathesis step from the dimerization and isomerization. The work within this project includes also the in-house preparation of the catalysts in a reproducible manner for the two-reactor concept, their characterization with modern techniques, the investigation of catalysts deactivation and the determination of reaction kinetics.
Characterization equipment
- Fully automated laboratory and miniplant multi-reactor equipment for catalytic testing and kinetic measurements.
- Powder XRD - X`Pert PRO apparatus (PANalytical) with CuKα.
- Nova 2000e (Quantachrome) surface area and pore size analyzer.
- Chem Star TPx+ (Quantachrome/3P Instruments) and TPD-R-O (Porotec) equipments (TPR, TPO, pulse chemisorption, TPD).
- FT-Raman Spectrometer - MultiRam (BRUKER Optics) for powder sample.
- Support by the Faculty of Natural Sciences, Institute of Experimental Physics and the Chemical Institute of the Otto-von-Guericke-University Magdeburg with TEM, EDX, FTIR and MAS-NMR.


References
Alvarado Perea, L., Felischak, M., Wolff, T., López Gaona, J. A., Hamel, C., & Seidel-Morgenstern, A. (2021). Propene production at low temperature by bimetallic Ni-Mo and Ni-Re catalysts on mesoporous MCM-41 prepared using template ion exchange. Fuel, 284, 119031.
Felischak, M., Wolff, T., Alvarado Perea, L., Seidel-Morgenstern, A., & Hamel, C. (2019). Influence of process parameters on single bed Ni/(Al)MCM-41 for the production of propene from ethene feedstock. Chemical Engineering Science, 210, 115246.
Felischak, M., Seidel-Morgenstern, A., Hamel, C. (2018). „Conceptual study of the ethene to propene reaction carried out in a reactor cascade”, Jahrestreffen Reaktionstechnik, Würzburg.
Felischak, M., Wolff, T., Alvarado-Perea, L., Seidel-Morgenstern, A., Hamel, C. (2018). „Kinetic Investigation of propene production under metathesis conditions applying W/SiO2”, 51. Jahrestreffen Deutscher Katalytiker, Weimar.
Alvarado-Perea, L., Felischak, M., Wolff, T., Hamel, C., Seidel-Morgenstern, A. (2017). „Experimental Investigation of the Reaction Network of Ethene to Propene over Ni/AlMCM-41 Catalysts”, Chemie-Ingenieur-Technik, 89, 903-914.
Wolff, T., Felischak, M., Alvarado-Perea, L., Lopez Goana, J.A., Hamel, C., Seidel-Morgenstern, A. (2017). „Deactivation study of Mo, W, Re and NiRe on mesoporous support for metathesis of ethene and 2-butene”, Jahrestreffen Reaktionstechnik, Würzburg.
Felischak, M., Wolff, T., Alvarado-Perea, L., Lopez Goana, J.A., Seidel-Morgenstern, A., Hamel, C. (2017). „Mechanistic Investigation of propene production from ethene feed for long time on stream using Ni/(Al)MCM-41”, Jahrestreffen Reaktionstechnik, Würzburg.
Wolff, T., Felischak, M., Alvarado-Perea, L., Lopez Goana, J.A., Hamel, C., Seidel-Morgenstern, A. (2017). „Experimental investigation of the metathesis of ethene and 2-butene using metallic catalysts supported on mesoporous material”, 50. Jahrestreffen Deutscher Katalytiker, Weimar.
Felischak, M., Wolff, T., Alvarado-Perea, L., Lopez Goana, J.A., Seidel-Morgenstern, A., Hamel, C. (2017). „Investigation of prolonged Ni/(Al)MCM-41 application under reaction conditions for the production of propene from ethene”, 50. Jahrestreffen Deutscher Katalytiker, Weimar.
Felischak, M., Wolff, T., Alvarado-Perea, L., Seidel-Morgenstern, A., Hamel, C. (2016). „Direct Synthesis of Propene from Ethene Feedstock: Investigation of Catalytic Concepts“, AIChE Annual Meeting San Francisco.
Wolff, T., Alvarado-Perea, L., Felischak, M., Hamel, C., Seidel-Morgenstern, A. (2016). „Direct transformation of ethene to propene: study of deactivation of catalysts for two-reactor concept”, Jahrestreffen Reaktionstechnik, Würzburg.
Hydroaminomethylation of long chain olefins
Kinetics of reductive amination and hydroaminomethylation in reactive multiphase fluid systems
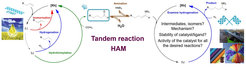
The Collaborative Research Center Transregio 63 of the German Research Foundation (DFG) develops methods and strategies to adapt established reactions to substrates from renewable raw materials, as illustrated in Figure 1. In the first two funding periods, the reaction network for the hydroformylation of long-chain olefins (1-dodecene, 1-decene) was identified, the catalytic cycles for all main and side reactions were developed and mechanistic kinetic models were derived. Focus of the third funding period is the evaluation of the potential of complex homogeneously catalyzed tandem reactions using the example of the rhodium-catalyzed hydroaminomethylation (HAM) which couples the hydroformylation with the reductive amination (RA). Thus, the gained knowledge and developed methods for the hydroformylation (HYFO) should be used to analyse and quantify the kinetics for the overall reaction system of the hydroaminomethylation.
References
Gerlach M., Kirschtowski S., Jameel F., Huxoll F., Stein M., Sadowski G., Seidel-Morgenstern A., & Hamel C. (2021). Kinetic Modeling of Complex Catalytic Reactions in Multiphase Systems, Chapter in the book written by the InPROMT consortium: Integrated Chemical Processes in Liquid Multiphase systems - From chemical reaction to process design and operation (Ed. Kraume M.), De Gruyter, Oldenbourg, to be submitted in 2021.
Kirschtowski, S., Jameel, F., Stein, M., Seidel-Morgenstern, A., & Hamel, C. (2021). Kinetics of the Reductive Amination of 1-Undecanal in Thermomorphic Multicomponent System. Chemical Engineering Science, 116187.
Kirschtowski, S., Kadar, C., Seidel-Morgenstern, A., & Hamel, C. (2020). Kinetic Modeling of Rhodium-Catalyzed Reductive Amination of Undecanal in Different Solvent Systems. Chemie-Ingenieur-Technik, 92(5), 582-588.
Gerlach, M., Kirschtowski, S., Seidel-Morgenstern, A., & Hamel, C. (2018). Kinetic Modeling of the Palladium-Catalyzed Isomerizing Methoxycarbonylation of 1-Decene. Chemie-Ingenieur-Technik, 90(5), 673-678.
![Figure 1: ETP reaction mechanism.[Felischak, M., Wolff, T., Alvarado Perea, L., Seidel-Morgenstern, A., & Hamel, C. (2019). Influence of process parameters on single bed Ni/(Al)MCM-41 for the production of propene from ethene feedstock. Chemical Engineering Science, 210: 115246.] Figure 1: ETP reaction mechanism.[Felischak, M., Wolff, T., Alvarado Perea, L., Seidel-Morgenstern, A., & Hamel, C. (2019). Influence of process parameters on single bed Ni/(Al)MCM-41 for the production of propene from ethene feedstock. Chemical Engineering Science, 210: 115246.]](/3553075/original-1636650272.jpg?t=eyJ3aWR0aCI6MzQxLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzU1MzA3NX0%3D--1723feb25634626fc43f2242a79cfe78ae2c7641)



