On the path to an artificial cell
With the integration of a rudimentary metabolic function into a tiny droplet, a step has been made towards advancing the borders of life
It is hoped that cells created in a test tube can answer some of the major questions in biology. What is the minimum that a cell needs in order to live? And how did life on Earth begin? Researchers from the Max Planck Institute for Dynamics of Complex Technical Systems in Magdeburg and the Paul Pascal Research Center at the CNRS and University of Bordeaux are now presenting the forerunners of an artificial cell. In an experiment of synthetic Biology they have succeeded in incorporating the simple form of a metabolic function into microscopically small droplets: a chemical reaction, maintained by an integrated energy supply.
Text; JD/PH, Max Planck Society
“How does a living organism avoid deteriorating?”, Erwin Schrödinger asks in his book, “What is Life?”, in which he explains the physical aspects of living matter. According to the physicist, the answer is simple: “Through eating, drinking and breathing (...)”. The specialist term used for this is “metabolism”, better known as “metabolic function”. The biochemical processes that occur enable living organisms to gain energy and build up or break down substances. For individual cells, too - regardless of whether they are single-cell organisms or are organized within a larger organism - metabolic function is essential for the ability to live and survive.
Living cells need a metabolism and a boundary to the environment
Therefore, if researchers in synthetic biology wish to synthesize cells, among other things, they must integrate a metabolism into a space that is separated off from the environment. This is precisely what scientists, led by Jean-Christophe Baret from the Centre de Recherche Paul Pascal (CRPP, in English: the Paul Pascal Research Centre) in Bordeaux and Kai Sundmacher from the Max Planck Institute for Dynamics of Complex Technical Systems in Magdeburg, have now succeeded in doing in a simplified form. Here, their artificial cells consisted of nothing other than microscopically small water droplets, which were formed in oil. They served the researchers as tiny units that were separated from their environment - similar to cells that are separated off from their environment by a membrane.
The researchers added different molecular components into the interior of these droplets, which in turn simulated a metabolic reaction. Admittedly, at first sight, such a simplified synthetic cell looks very different from its natural equivalent. However, one thing is certain: “From a technological perspective, such minimal systems are relevant models from which more complex systems that are closer to nature can be developed”, Kai Sundmacher, Director of the Max Planck Institute in Magdeburg explains.
What are the decisive components for a living cell?
According to Ivan Ivanov, engineer and researcher at the Max Planck Institute for Dynamics of Complex Technical Systems, he and his colleagues initially anyway only wanted to design a minimal system that has the basic properties of the cell. This is the only way that makes it possible to find out which components are ultimately of decisive importance for life. Step by step, he and his colleagues therefore built a model metabolic function from molecular components. The jargon used by specialists for this procedure is the bottom-up principle.
For engineers, the bottom-up approach is part of their everyday work, but for synthetic biologists, it is not. Instead, they usually work using the top-down principle. They start with a real organism, which they modify using genetic technology methods, thus equipping it with new functions and properties. “In the genetic material of cells, however, there are many things that are redundant or even unnecessary”, Ivanov explains, with reference to the problem of using top-down approaches. After all, in such cases, the scientists do not learn which features really are necessary for the creation of life.
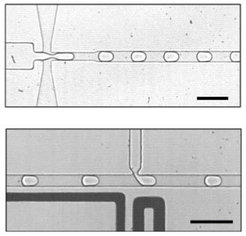
The microfluidic technique produces droplets as required
As well as the metabolic function, separation from the environment is also needed. As Ivanov explains, “Each cell has a wall to a certain extent, which separates it from its environment”. Such separate compartments, as the specialists call them, can either be created through membranes or, as in this current work, through droplets.
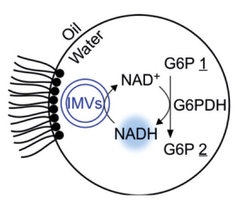
The researchers are using what is known as “microfluidic technology”, which makes it possible to produce microdroplets in large numbers and quickly analyze them. Here, the scientists have been able to finely adjust both the size and the composition as required. Using microfluidic modules, they then filled the compartments with glucose phosphate and the co-factor NAD+. To a certain extent, the former provides nutrients for the artificial cells, which in the presence of the co-factor NAD+ are transformed into a chemical end product while releasing chemical energy.
NAD+ also plays a role in the metabolism of living cells, and absorbs hydrogen during the course of the metabolic reaction, so that it is converted into NADH. In order for the reaction to be maintained in reality, the scientists added a module that regenerates the NAD+ by oxidizing NADH back to NAD+. Thus, the co-factor is always available in its required form.
If the glucose phosphate has been entirely used up, the cells go into a sleep mode to a certain degree, which could be brought to an end through renewed feeding with their nutrients, using – again – a microinjection system.
Real cells must multiply and store their structural design
According to the head of the project, Jean-Christophe Baret, the model metabolism has all the basic features of natural metabolic function and offers a platform for further studies: “With the microfluidic technology, we can produce controlled quantities of such elementary components and give them even more complex functions. In this way, hypotheses can in turn be tested regarding the creation of life from known and controlled ingredients.” In order to really imitate genuine cells in a way that is sufficiently close to reality, such systems also require the ability to reproduce, for example, as well as a mechanism for storing their structural design, a set of features still ahead of us.
However, even without these features, for the lead author of the publication, Thomas Beneyton, it is possible that such artificial systems will behave in a similar way to biological ones. For example, droplets can be produced with “unequal fitness” - in other words, with a different appetite or with a variable output quantity of nutrients - and permit the exchange of nutrients among the cells. In this way, a competition situation could be created such as those that are also observed among real cells. Such droplet cells would then behave entirely in accordance with Darwin’s theory.

