
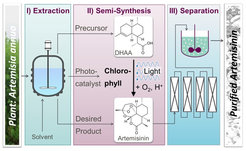
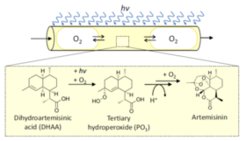
Reaction systems and process variants
References
Triemer, S., Schulze, M., Wriedt, B., Schenkendorf, R., Ziegenbalg, D., Krewer, U., & Seidel-Morgenstern, A. (2021). Kinetic analysis of the partial synthesis of artemisinin: Photooxygenation to the intermediate hydroperoxide. Journal of Flow Chemistry. Journal of Flow Chemistry. 11, 641-659.
Horosanskaia, E., Triemer, S., Seidel-Morgenstern, A., & Lorenz, H. (2019). Purification of Artemisinin from the Product Solution of a Semisynthetic Reaction within a Single Crystallization Step. Organic Process Research & Development, 23, 2074-2079.
Münzberg, S., Vu, T. G., Seidel-Morgenstern, A. (2018). Generalizing Countercurrent Processes: Distillation and Beyond, Chemie Ingenieur Technik, 90 (11), 1769-1781.
Triemer, S., Gilmore, K., Vu, T.G., Seeberger, P.H., Seidel-Morgenstern, A. (2018). Literally Green Chemical Synthesis of Artemisinin from Plant Extracts, Angewandte Chemie International Edition, 57 (19), 5525-5528.
Horvath Z., Horosanskaia E., Lee J. W., Lorenz H., Gilmore K., Seeberger P.H., Seidel-Morgenstern, A. (2015). Recovery of artemisinin from a complex reaction mixture using continuous chromatography and crystallization, Organic Process Research & Development, 19 (6), 624-634.
Horvath, Z., O'Brien, A. G., Seeberger, P. H., Seidel-Morgenstern, A. (2014). Design and optimization of coupling a continuously operated reactor with simulated moving bed chromatography. Chemical Engineering Journal , 251, 355-370.
Gilmore K., Kopetzki, D., Lee J.W., Horváth Z., McQuade D.T., Seidel Morgenstern A., Seeberger P.H. (2014). Continuous synthesis of artemisinin-derived medicines, Chem. Comm, 50, 12652-12655.
Coupled enantioselective resolution and enzymatic racemization
Keywords: enantioselective HPLC, preferential crystallization, enzymatic racemization, mathematical modelling.
While significant improvement is made in enantioselective resolution techniques, such processes suffer a major drawback in their 50% yield constraint, as the unwanted by-product enantiomer is typically discarded after the separation. An attractive solution is the subsequent recycling of the by-product enantiomer by racemization. The racemization reaction converts the by-product into a racemic mixture of both enantiomers, which can be separated again, resulting in a significant yield increase.
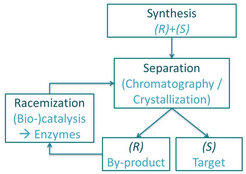
Racemization reaction
Racemization can be performed chemically, but this process often includes high temperatures, extreme pH values or is very slow. An eco-friendly alternative is the use of enzymes as biocatalyst, which not only accelerate the racemization reaction but in general also operate at moderate conditions, allowing an easier coupling with the resolution units.
Coupling options
In this group, model molecules are separated with enantioselective liquid chromatography (LC) and with preferential crystallization (PC) followed in both cases a by-product racemization with an immobilized racemase. The process with LC was studied for resolving the mandelic acid enantiomers using a mandelate racemase. For comparison, the coupled process with PC was tested for resolution of the enantiomers of the conglomerate forming amino acid asparagine coupled with an amino acid racemase catalyzed reaction. Suitable initial operating conditions for the process couplings were identified empirically for the single process units as well as for the coupled processes.
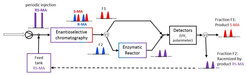
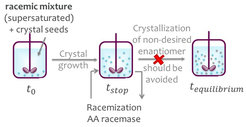
Enzyme immobilization
The enzyme production is often time consuming and can be expensive. Therefore, the enzyme is immobilized to enable reuse. Suitable immobilization procedures were developed for both enzymes. The amino acid racemase was purified with metal ion affinity chromatography and immobilized on Lifetech amino-activated resin. Due to its high activity, the crude extract of mandelate racemase was directly immobilized on Eupergit CM by Sigma-Aldrich.
Process simulation
Simulations based on mathematical models and experimental parameters were used for a deeper process understanding and to investigate the influence of operating conditions on the key performance indicators. In both cases the coupling not only increased the coupled process yield, but also its the purity and productivity. All obtained information were evaluated to find optimal conditions and to identify the potential of the two variants of the coupled process for resolution of enantiomers of other chiral compounds.
References
Harriehausen, I., Wrzosek, K., Lorenz, H., & Seidel-Morgenstern, A. (2020). Assessment of process configurations to combine enantioselective chromatography with enzymatic racemization. Adsorption, 26, 119-1213.
Carneiro, T., Wrzosek, K., Bettenbrock, K., Lorenz, H., & Seidel-Morgenstern, A. (2020). Immobilization of an amino acid racemase for application in crystallization‐based chiral resolutions of asparagine monohydrate. Engineering in Life Sciences, 20, 550-561.
Wrzosek, K., Harriehausen, I., & Seidel-Morgenstern, A. (2018). Combination of enantioselective preparative chromatography and racemization: Experimental demonstration and model-based process optimization. Organic Process Research &Development, 22(12), 1761-1771.




