Development of a Multiplex Analysis for the Optimization of Cell-Culture-Based Vaccine Production
Motivation
The project described here is a multidisciplinary study for understanding the complex interactions between influenza A viruses (IAVs) and host cells using state-of-the-art imaging flow cytometry and in situ detection methods to investigate viral RNA and protein dynamics on the molecular level. Characterization of virus replication dynamics will focus on two key technologies: (i) a fluorescence assay using nuclease-resistant molecular beacons (MBs, Fig. 1) with high specificity and sensitivity for early detection of IAVs and for real-time monitoring of virus propagation; and (ii) imaging flow cytometry, which combines the analytical power of conventional flow cytometry with the visual detail of imaging to resolve the spatial-temporal distribution of IAV proteins and RNAs during the progress of infection (Fig. 2). The overall objectives of this research are to provide fundamental insights into virus proliferation and to apply this knowledge to derive new strategies to optimize the cell-culture-based influenza vaccine production.
Aims
- Nuclease-resistant MB-based fluorescent-probes will be designed to target a highly conserved internal region on the IAV genome; the MB-based fluorescence assays will be performed to study the IAV infection dynamics in single cells and cell populations (MDCK, HEK293, etc.), especially highlighting key events in early infection, vRNA packaging, assembly and budding of progeny virions.
- The established MB detection method enables qualitative observations to provide a starting point for systems biology approaches to establish and validate models describing complex virus-host interactions. Moreover, the MB-based detection will be combined with the existing immunostaining methods and reveal details in the IAV life cycle at both the vRNA and protein level.
- The fluorescence assay using imaging flow cytometry greatly reduces quantification time and produces infectivity information as a function of p.i. time points. Results obtained will be interpreted in the context of low virus yields, cell-to-cell spreading of virions, and technical challenges of low and high density cell cultured-based IAV vaccine production. Ultimately, the assay will support studies where the quantity of virus particles (yield) in upstream processing is an important factor, such as the production of viral vaccines.
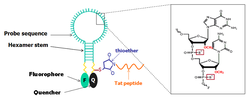
Figure 1. Schematic representation of the nuclease-resistant MB-Tat. The phosphodiester bond is modified by replacing non-bridging oxygen with sulfur and the 2′-sugar deoxy with 2′-O-methyl group. The thiol group at the quencher end reacts with a maleimide group placed at the N terminus of the Tat-peptide to yield a chemically stable thioether bond.
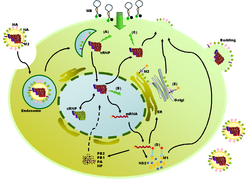
Figure 2. Schematic diagram of the IAV life cycle and detection of viral replication by introducing MBs and mAbs. MBs report the presence of the IAV by forming the fluorescent hybrids with vRNAs during the course of viral reproduction, such as: (A) the release of vRNPs into the cytoplasm, (B) RNA replication in the nucleus, and (C) the nucleus export of the newly-synthesized vRNPs. The vRNP binding activities of the matrix proteins and the M1-M2 interactions are shown by immunofluorescent staining with mAbs against M1 (D) or M2 (E). ER, endoplasmic reticulum.

