Cancer and Inflammation
There is recent evidence that inflammation is a critical component in tumor progression. Many cancerous cells arise from sites of infection, chronic irritation and inflammation. It is now becoming clear that the cellular microenvironment has an important role and additionally tumor cells have co-opted some of the signaling molecules of the immune system such as the nuclear growth factor NF-κB. We are here aiming at an understanding of the structural biology and the link between inflammation and cancer.
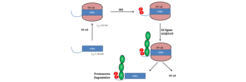
A structural model of the N-terminal signal-receiving Domain (SRD) of the NF-ΚB inhibitor IΚBα was refined from a combination of protein threading and long term molecular dynamics simulations. The SRD model was found to be stable for the long living NF-ΚB -IΚBα protein-protein complex and free IΚBα in solution. The signal‐receiving N‐terminal domain (SRD) of the NF‐κB inhibitor IκBα harbors the sites of post‐translational modifications (Ser32 and 36) directed by the IκB kinase (IKK) complex. Identifying and characterizing the structural effects that arise from phosphorylation may explain how phosphorylation regulates the IκBα‐NF‐κB protein complex.

The structural modifications of the IκBα‐NF‐κB protein‐protein complex due to mono‐phosphorylation of either Ser32 or Ser36 amino acid residues or simultaneous phosphorylation were investigated by means of molecular dynamics simulations. Only this two‐fold phosphorylation induced an extended, but more stabilized conformation of the degron motif which renders it accessible by the E3 ligase. In summary, these results provide insight into the conformational changes induced in IκBα proteins upon phosphorylation that are vital to their signaling dynamics and enable us to propose a model for the phosphorylation of the SRD.

