Upstream Processing
Innovation for modern biotechnological processes will be essential to keep the medical supply affordable for a still increasing world population. New vaccines for pandemics or emerging diseases, therapeutic vaccines, recent developments in gene therapy, and options for cancer treatment (antibody-drug-conjugates), but equally global travelling, global distribution of goods, constant zones of war or terror and aging societies demand for efficient approaches in manufacturing of biologicals to cope with supply needs.Depending on the application many doses (vaccines) or doses with high virus concentration (virotherapy) will be needed, ultimately demanding highly efficient virus production processes to cover the future needs.
Therefore, ideas for innovative products and new approaches for their production on a small scale have to be transformed into complete process trains at a large scale. Production costs, working volumes, effective scale-up, foot print of plants, product yield, downstream processing and many other issues have to be optimized before a process will be used in pharmaceutical industry. Accordingly, we focus on optimization, intensification and integration of bioprocesses on different levels. These include host cell selection, medium design, cultivation parameters, feeding strategies, dynamics of cell growth and product formation as well as approaches towards efficient high cell density cultures and continuous cultivation. In addition, properties of cell lines, specific aspects of cellular growth and metabolism, virus replication, and recombinant protein expression are characterized in detail.
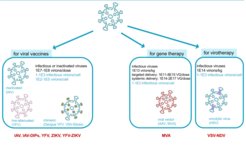
Our focus is on virus particles and viral vectors for vaccination and therapy. Currently, we mainly consider production systems for influenza A virus (IAV), modified vaccinia virus Ankara (MVA) virus, and various flaviviruses such as yellow fever virus (YFV), Japanese Encephalitis virus (JEV), and Zika virus (ZIKV). Moreover, we evaluate cell lines for MLV vector production or production of oncolytic viruses (based on vesicular stomatitis virus (VSV) and Newcastle disease virus (NDV) (Fig. 1). Therefore, growth and product formation of various adherent as well as suspension host cells are being evaluated.