
ALLS: Artificial Life-Like Systems
The exciting field of Synthetic Biology focuses on producing engineered, predictable cells or other biological systems to carry out desired functions, whereby these functions can be modified, enhanced or entirely new. Conceptually, Synthetic Biology relies on breaking down the complexity of biological systems into smaller subsystems and functional modules, followed by (re-)designing and (re-)assembling them into larger units. This can be considered as an analogue to the design of a biotechnological or chemical plant (Rollié et al., 2012). With respect to this, the PSE Group, in collaboration with EEC Group of Dr. Tanja Vidaković-Koch, had introduced its expertise in the assembly and control of chemical, energy and production processes in MaxSynBio– a research consortium with eight other Max Planck Institutes and research groups from the Friedrich-Alexander University in Erlangen-Nuremberg and the University of Bordeaux, which was jointly funded by the Max Planck Society and the Federal Ministry of Education and Research in Germany.
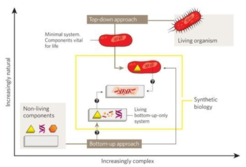
The primary scientific goal of MaxSynBio was a true bottom-up engineering approach for mimicking the fundamental structural and functional principles of real cells towards better understanding of living systems (Fig. 1). The near future in Synthetic Biology will still lean heavily on the top-down approach, i.e. minimal systems derived from an existing organism. The bottom-up approach however – starting from first principles – might allow us to refine and further improve these minimal systems with alternative synthetic or biological components and pave the way for establishing a radically new generation of biotechnological production processes.
The MaxSynBio consortium focused on selected life processes, which are of fundamental importance for the replication and functionality of living cells, such as growth, division, morphogenesis, signaling and motility, summarized under the umbrella term “proliferome” (Schwille et al., 2018). The specific responsibilities of the PSE group within the MaxSynBio consortium included:
- Compartmentalization and growth (tools for production, functionalization, characterization, and controlled increase of compartment size)
- Establishment and characterization of energy and cofactor regeneration modules for artificial cells (light- or chemically-driven)
- Establishment and characterization of metabolic (e.g. for CO2 fixation) and transport modules
- Coupling of the energy supply modules with different energy sink subsystems
- General methodology for integration of functional parts and modules and modular design of synthetic cells, based on assembly standards and mathematical modeling
and the general workflow is depicted in Fig. 2.

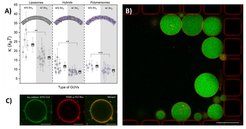
Compartmentalization is a major landmark of living systems – to establish gradients for energy generation or to spatially segregate metabolic reactions from the environment – and therefore the reconstitution of living processes is inherently associated with the creation and manipulation of compartments. Phospholipid membranes closely imitate the architecture and function of natural membranes and they have been widely used in planar (e.g. as supported bilayers) and closed (vesicles) architectures as model systems. Recently, amphiphilic copolymer-based analogues of cellular membranes have expanded the chemical dimensions of supramolecular structures, which allows for further tailoring to technological, biological and medical applications. Within the compartmentalization technology platform, we are developing various methods for production and characterization of nano- and microcompartments of different chemical compositions, including conventional and microfluidic tools (Weiss et al., 2018). In addition, we establish different protocols for membrane functionalization and studying the respective interactions (e.g. with membrane proteins) (Fig. 3, Marušič et al., 2020).
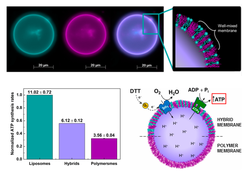
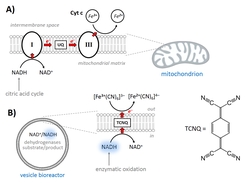
Active processes in living systems need continuous supply of energy and materials and the energy supply and storage are closely connected to the cellular metabolism. Following the bottom-up approach, we have obtained and characterized the basic biochemical machinery for energy transduction and conversion – light- and chemically-driven proton pumps and ATP synthase – and are constantly expanding the library of purified membrane proteins (other oxidases, porins, fusogenic peptides, etc.). We are integrating energy-converting parts in three types of nano- and microcompartments – liposomes, hybrid vesicles and polymersomes – and characterizing them with respect to activity, reconstitution efficiency and orientation, whereby experimental optimization resulted in retention of enzymatic activity in semisynthetic and synthetic nanocompartments (Fig. 4, Otrin et al., 2017). We have also demonstrated encapsulated cofactor regeneration by a hydrophobic redox mediator as a synthetic analog for NADH dehydrogenase and cytochrome c reductase (Fig. 5, Wang et al., 2018). The membrane proteins insertion optimization requires rigorous screening for the ideal reconstitution conditions and, more often than not, the latter are found to differ significantly between the various enzymes. Simultaneous integration of several enzymes via fusion can be exploited in order to circumvent those limitations. SNARE fusogenic proteins were explored in this context, and their fusion intermediates of polymer and hybrid membranes were proposed (Fig. 6, Otrin et al., 2021). Furthermore, we are exploring various metabolic pathways and ways to integrate them with energy supply modules. In parallel, we use the respiratory chain from E. coli membranes as a natural counterpart of bottom-up-assembled modules and combine it with a simple metabolism (Beneyton et al., 2018). Additionally, we describe the energy and metabolic modules through mathematical models, which provides possibilities for optimization and a quantitative framework for their integration.

Following the success of MaxSynBio, PSE group is now continuing with construction of artificial cells under the new initiative, i.e., ALLS (Artificial Life-Like Systems). Toward this end, SynBio researches in PSE redefined minimal functionalities required for artificial life-like system, with emphasis on their compatibility. Those functionalities comprise motility, sensing/signaling, metabolism and energy. Motility will enable artificial cell to move in response to the environment (integration with sensing/signaling), thereby mimicking an invaluable aspect of living matter. Energy, in the form of ATP, required for activation of motility module, will be supplied via metabolic cascade and ATP-generating module. Enabling technologies, such as compartmentalization, transport, cell-free protein expression and membrane fusion are being further developed. For example, in addition to SNARE-mediated fusion, we explored also mechanically-induced salt-mediated fusion (Marušič et al., 2022) and fusion based on electrostatic interactions between oppositely charged membranes (Marušič et al., 2022).
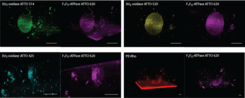
Micro-sized life-like systems are characterized by our new confocal microscope Leica STELLARIS 5: analysis of integration, partitioning (Fig. 7), orientation, lateral diffusion and functionality of membrane proteins (monitoring pH, ATP and substrates changes).
Recent Publications
Otrin N., Otrin L., Bednarz C., Träger T. K., Hamdi F., Kastritis P. L., Ivanov I., and Sundmacher K. (2024). Protein-rich rafts in hybrid polymer/lipid giant unilamellar vesicles. Biomacromolecules
Marušič N., Otrin L., Rauchhaus J., Zhao Z., Kyrilis F.L., Hamdi F., Kastritis P.L., Dimova R., Ivanov I., and Sundmacher K. (2022). Increased efficiency of charge-mediated fusion in polymer/lipid hybrid membranes. Proceedings of National Academy of Sciences 119(20): e2122468119
Marušič N., Zhao Z., Otrin L., Dimova R., Ivanov I., and Sundmacher K. (2022). Fusion-induced Growth of Biomimetic Polymersomes: Behavior of poly(dimethylsiloxane)-poly(ethylene oxide) Vesicles in Saline Solutions Under High Agitation. Macromolecular Rapid Communications 43(5): e2100712
Staufer O., De Lora J. A., Bailoni E., Bazrasfhan A., Benk A. S., Jahnke K., Manzer Z. A., Otrin L., Pérez T. D., Sharon J., Steinkühler J., Adamala K. P., Jacobson B., Dogterom M., Göpfrich K., Stefanovic D., Atlas S. R., Grunze M., Lakin M. R., Shreve A. P., Spatz J. S., and López G. P. (2021). Science Forum: Building a community to engineer synthetic cells and organelles from the bottom-up. eLife 10: e73556
Otrin L., Witkowska A., Marušič N., Zhao Z., B. Lira R., Kyrilis F., Hamdi F., Ivanov I., Lipowsky R., L. Kastritis P., Dimova R., Sundmacher K., Jahn R., and Vidaković-Koch T. (2021). En route to dynamic life processes by SNARE-mediated fusion of polymer and hybrid membranes. Nature Communications 12: 4972
Shetty C. S., Yandrapalli N., Pinkwart K., Krafft D., Vidakovic-Koch T., Ivanov I., and Robinson T. (2021). Directed Signaling Cascades in Monodisperse Artificial Eukaryotic Cells. ACS Nano 15: 15656−15666
Balasbas III S., Ivanov I. and Sundmacher K. (2021). Metabolic Network Reconstruction from Time-Series of Concentration Data: Evaluation of the Numerical Matrices Methods. Computer Aided Chemical Engineering 50: 1991-1996
Ivanov I., López Castellanos S., Balasbas III S., Otrin L., Marušič N., Vidaković-Koch T., and Sundmacher K. (2021). Bottom-Up Synthesis of Artificial Cells: Recent Highlights and Future Challenges. Annual Review of Chemical and Biomolecular Engineering 12: 287-308
Wang M., Weber A., Hartig R., Zheng Y., Krafft D., Vidaković-Koch T., Zuschratter W., Ivanov I., Sundmacher K. (2021). Scale up of transmembrane NADH oxidation in synthetic giant vesicles. Bioconjugate Chemistry 32 (5): 897–903.
Ahmad R., Kleineberg C., Nasirimarekani V., Su Y.-J., Pozveh S. G., Bae A., Sundmacher K., Bodenschatz E., Guido I., Vidaković-Koch T., and Gholami A. (2021). Light-Powered Reactivation of Flagella and Contraction of Microtubule Networks: Toward Building an Artificial Cell. ACS Synthetic Biology 10 (6): 1490–1504
Wohlfromm F., Richter M., Otrin L., Seyrek K., Vidaković-Koch T., Kuligina E., Richter V., Koval O., and Lavrik I. N. (2021). Interplay Between Mitophagy and Apoptosis Defines a Cell Fate Upon Co-treatment of Breast Cancer Cells With a Recombinant Fragment of Human κ-Casein and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Frontiers in Cell and Developmental Biology 8: 617762
Xu D., Kleineberg C., Vidaković-Koch T., Wegner S. V. (2020). Multistimuli sensing adhesion unit for the self-positioning of minimal synthetic cells. Small 16 (35): 2002440
Marušič N., Otrin O., Zhao Z., Lira R.B., Kyrilis F.L., Hamdi F., Kastritis P.L., Vidaković-Koch T., Ivanov I., Sundmacher K., and Dimova R. (2020). Constructing artificial respiratory chain in polymer compartments: Insights into the interplay between bo3 oxidase and the membrane. Proceedings of National Academy of Sciences 117 (26): 15006-15017
Kleineberg C., Wölfer C., Abbasnia A., Pischel D., Bednarz C., Ivanov I., Heitkamp T., Börsch M., Sundmacher K., Vidaković-Koch T. (2020). Light-driven ATP regeneration in diblock/grafted hybrid vesicles. ChemBioChem 21 (15): 2149-2160
Otrin L., Kleineberg C., de Silva L.C., Landfester K., Ivanov I., Wang M, Bednarz C., Sundmacher K., Vidaković-Koch T. (2019). Artifical organelles for energy regeneration. Advanced Biosystems 3 (6): 1800323
Ivanov I., Lira R.B., Tang T.-Y.D., Franzmann T., Klosin A., de Silva L.C., Hyman A., Landfester K., Lipowsky R., Sundmacher K., and Dimova R. (2019). Directed growth of biomimetic microcompartments. Advanced Biosystems 3: 1800314
Krafft D., López Castellanos S., Lira R.B., Dimova R., Ivanov I., Sundmacher K. (2019). Compartments for synthetic cells: Osmotically assisted separtion of oil from double emulsions in microfluidic chip. ChemBioChem 20: 2604-2608
Beneyton T., Krafft D., Bednarz C., Kleineberg C.,Woelfer C., Ivanov I., Vidaković-Koch T., Sundmacher K., and Baret J. C. (2018). Out-of-equilibrium microcompartments for the bottom-up integration of metabolic functions. Nature Communications 9: 2391
Wang M., Wölfer C., Otrin L., Ivanov I., Vidaković-Koch T., and Sundmacher K. (2018). Transmembrane NADH Oxidation with Tetracyanoquinodimethane. Langmuir 34 (19): 5435-5443
Weiss M., Frohnmayer J. P., Benk L. T., Haller B., Janiesch J. W., Heitkamp T., Börsch M., Lira R. B., Dimova R., Lipowsky R., Bodenschatz E., Baret J. C., Vidakovic-Koch T., Sundmacher K., Platzman I., Spatz J. P. (2018). Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nature Materials 17(1): 89-96
Schwille P., Spatz J., Landfester K., Bodenschatz E., Herminghaus S., Sourjik V., Erb T. J., Bastiaens P., Lipowsky R., Hyman A., Dabrock P., Baret J. C., Vidaković-Koch T., Bieling P., Dimova R., Mutschler H., Robinson T., Tang T. D., Wegner S., and Sundmacher K. (2018). MaxSynBio: Avenues Towards Creating Cells from the Bottom Up. Angewandte Chemie International Edition 57 (41):13382-13392
Otrin L., Marušič N., Bednarz C., Vidaković-Koch T., Lieberwirth I., Landfester K., and Sundmacher K. (2017). Toward Artificial Mitochondrion: Mimicking Oxidative Phosphorylation in Polymer and Hybrid Membranes. Nano Letters 17 (11): 6816-6821
Rollié S., Mangold M. and Sundmacher K. (2012). Designing biological systems: systems engineering meets synthetic biology. Chemical Engineering Science, 69 (1): 1-29
Related Links
A step towards synthetic life (2021): https://bioengineeringcommunity.nature.com/posts/a-step-towards-synthetic-life
Artificial Mitochondrion, Bottom-up! (2021): https://www.youtube.com/watch?v=-RZdhF_X2W0
Charge vs. SNARE-mediated fusion of biomimetic polymer/lipid hybrid compartments: Which one is more efficient? (2021): https://www.youtube.com/watch?v=s0cSmIih6SA
Engineering Functional Modules for Life-like Systems: Energy Supply, Metabolism, and Growth (2021): https://mattertolife.cloud.panopto.eu/Panopto/Pages/Viewer.aspx?id=6241c32a-71a0-4394-b447-acd901053245
Max Planck School Matter to Life: https://www.youtube.com/watch?v=WWvuAEn5GCk, https://mattertolife.maxplanckschools.org/
Collaborations
Dr. Tanja Vidaković-Koch, Max Planck Institute for Dynamics of Complex Technical Systems, Magdeburg
Dr. Rumiana Dimova, Prof. Reinhard Lipowsky, Max Planck Institute of Colloids and Interfaces, Potsdam, Germany
Prof. Jean-Christophe Baret, University of Bordeaux, Bordeaux, France
Prof. Tobias Erb, Max Planck Institute for Terrestrial Microbiology, Marburg, Germany
Prof. Katharina Landfester, Max Planck Institute for Polymer Research, Mainz, Germany
Prof. Reinhard Jahn, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany
Prof. Robert J. Flassig, Brandenburg University of Applied Sciences, Brandenburg, Germany
Prof. Ramin Golestanian, Max Planck Institute for Dynamics and Self-Organization, Göttingen, Germany
Prof. Michael Börsch, Jena University Hospital, Jena, Germany
Prof. Panagiotis L. Kastritis, Martin Luther University Halle-Wittenberg, Halle, Germany
Prof. Seraphine Wegner, Institute of Physiological Chemistry and Phatobiochemistry, Münster, Germany
Prof. Eberhard Bodenschatz, Max Planck Institute for Dynamics and Self-organization, Göttingen, Germany
Prof. Marc Nowaczyk, Ruhr University Bochum, Bochum, Germany






