Continuous Purification of Influenza Virus
Motivation
Batch chromatography still is the work horse for the purification of many valuable products. Application in preparative scale, however, holds several disadvantages, like low productivity and high consumption of stationary and mobile phase.
To overcome those difficulties continuous chromatography such as simulated moving bed (SMB) technology can be employed. Those processes are already well-established for the separation of small molecules in chemical and pharmaceutical industry. Lately, the application of this technology for the purification of biotechnological molecules from complex mixtures has also been increasingly studied.
Aim of the project
The goal of the project is the establishment of continuous processes for the purification of cell culture-derived influenza virus based on SMB technology.
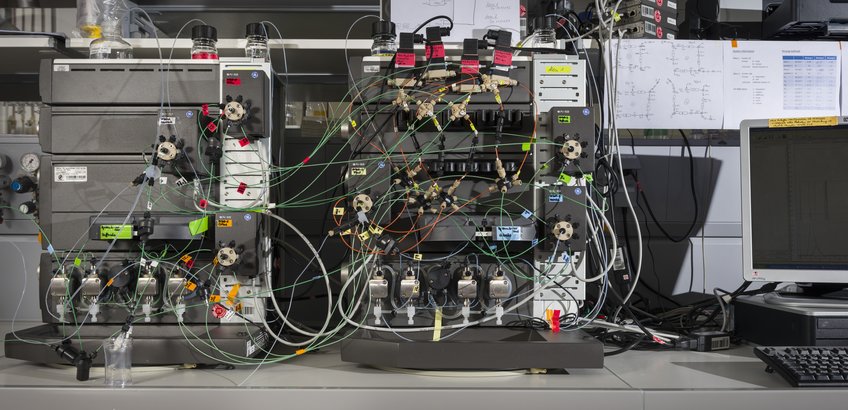
Simulated Moving Bed Technology

SMB processes are characterized by the countercurrent contact of mobile and stationary phase. The binary mixture containing the components to be separated is injected continuous into the system (Figure 1). The less adsorbing component (A) is transported by the mobile phase in one direction, while the stronger adsorbing component (B) is transported in the countercurrent direction by the stationary phase. Thus it is possible to remove both components at different outlet ports (extract and raffinate) continuously in high purity.
