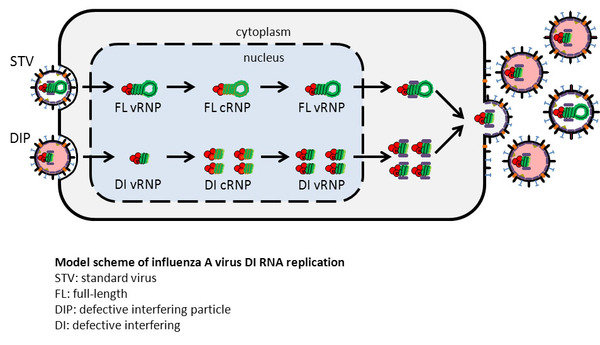
The aim of this project is to understand the dynamics of influenza A virus DIP replication. In particular, we want to identify factors involved in DIP generation, study the mechanism of interference on a molecular level, and analyze the impact of DIP formation on cell-specific virus yields. The findings can lead to new options to increase process yields in influenza vaccine production.
Additionally, we investigate options for cell culture-based production of the influenza A virus DIP DI244 (Wasik et al. 2017), which has been previously described as a potential candidate for influenza antiviral therapy. Cell-culture has several advantages to the conventional egg-based production, for example it is better scalable and enables full process control in closed systems. Our goal is the production of high yields of DI244 in chemically defined suspension culture.